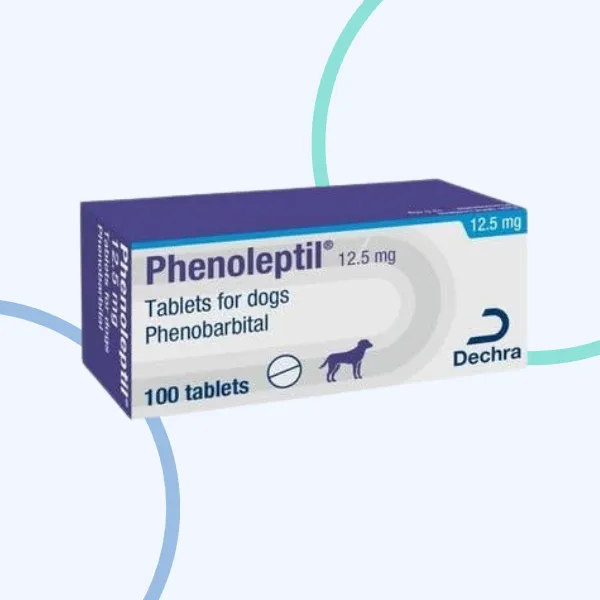
Phenoleptil Tablets
- Categories: Vet & Pet Pharmacy Dog's
Product & Safety Notice
- The maximum purchase for this product is 1.
- 3 days use only. This product can cause addiction.
- Click here for advice.
Guaranteed Safe Checkout
Phenoleptil Tablets
Indications for use
Prevention of seizures due to generalized epilepsy in dogs.
Contraindications
Do not use in case of hypersensitivity to the active substance or to other barbiturates.
Do not use in animals with serious impaired hepatic function.
Do not use in animals with serious renal or cardiovascular disorders.
Phenoleptil 12.5 mg: Do not use in dogs weighing less than 5 kg body weight.
Phenoleptil 25 mg: Do not use in dogs weighing less than 2.5 kg body weight.
Phenoleptil 100 mg: Do not use in dogs weighing less than 10 kg body weight.
Special warnings for each target species
The decision to start antiepileptic drug therapy with phenobarbital should be evaluated for each individual case and depends on number, frequency, duration and severity of seizures in dogs.
General recommendations for initiating therapy include a single seizure occurring more than once every 4-6 weeks, cluster seizure activity (i.e. more than one seizure within 24 hours) or status epilepticus regardless of frequency.
Some of the dogs are free of epileptic seizures during the treatment, but some of the dogs show only a seizure reduction, and some of the dogs are considered to be non-responders.
Special precautions for use in animals
(Phenoleptil 12.5 mg: These tablets should not be divided).
Doses for smaller dogs cannot be adjusted in accordance with the recommended 20% regime, and therefore special care should be taken in monitoring these animals. Also see Amounts to be administered and administration route.
Withdrawal of phenobarbital or transition to or from another type of antiepileptic therapy should be made gradually to avoid precipitating an increase in the frequency of seizures.
Caution is recommended in animals with impaired hepatic and renal function, hypovolemia, anaemia and cardiac or respiratory dysfunction.
Before beginning the treatment monitoring of hepatic parameters should be performed.
The chance of hepatotoxic side effects can be diminished or delayed using an effective dose that is as low as possible. Monitoring of hepatic parameters is recommended in case of a prolonged therapy.
It is recommended to assess the clinical pathology of the patient 2-3 weeks after start of treatment and afterwards every 4-6 months, e.g. measurement of hepatic enzymes and serum bile acids. It is important to know that the effects of hypoxia can cause increased levels of hepatic enzymes after a seizure. Phenobarbital may increase the activity of serum alkaline phosphatase and transaminases. These may demonstrate non-pathological changes, but could also represent hepatotoxicity, so liver function tests are recommended. Increased liver enzyme values may not always require a dose reduction of phenobarbital if the serum bile acids are in the normal range.
In the light of isolated reports describing hepatotoxicity associated with combination anticonvulsant therapy, it is recommended that:
1. Hepatic function is evaluated prior to initiation of therapy (e.g. measurement of serum bile acids).
2. Therapeutic phenobarbital serum concentrations are monitored to enable the lowest effective dose to be used. Typically concentrations of 15-45µg/ml are effective in controlling epilepsy.
3. Hepatic function is re-evaluated on a regular (6-12 months) basis.
4. Seizure activity is re-evaluated on a regular basis.
Please Note:
As this medication is a Schedule Three Drug, we require the original prescription posting to us


 Weight Loss
Weight Loss