- Home
-
-
-
-
-
-
-
-
- Skincare
- Sleep
- Buy Sleeping Tablets
- Migraine
- Diarrhoea & Constipation
- Threadworms
- Trapped Wind
- Haemorrhoids/Piles & Anal Fisure
- Dandruff
- Fungal Infections
- Scars, Cellulite & Stretch Marks
- Warts & Verrucas
- Cough & Cold
- Excessive Sweating
- Lice & Scabies
- Snoring
- Children & Babies
- Nausea
- Managing Medication - Pill Boxes And Tablet
- Acid Reflux & Heartburn
-
- Feminine Underwear
- Angina And Heart Health
- Children's Healthcare
- Cystitis & Urinary Infections
- Coughs, Cold And Flu
- Hair Care - Head Lice & Nits
- Skin Care.
- Hangover Relief
- Hayfever & Allergy Relief
- Health Monitoring & Diabetes
- Herbal Remedies
- Excessive Sweating
- Lip Care
- Nasal Care.
- First Aid
- Oral Health
- Ostomy Care & Catheter Care
- Pain Relief Care
- Piles & Haemorroids
- Sleeping Aids
- Thrush Treatments
- Travel Health
- Vaginal Infections
- Vertigo
- Digestion & Indigestion
- Toiletries
- Incontinence Care
- Low Protein
- Multivitamins & Supplements
- Baby Milk & Formula's
- Wound Management
- Diabetes Test Strips & Needles
- Feminine Hygiene
- Male Enhancement
- Eye Care
- Ear Care
- Mouth Care
- Foot Care
- Health Care
- Oral Trush
- Sun Care.
- Acne Care
- Rosacea Skin Care
- Psoriasis Skin Care
- Eczema Skin Care
- Hair Loss Care
- Quit Smoking Aids
- Asthma Healthcare
- CBD & Herbal Remedies
- Immunity
- Bone
- Nutritional Drinks
- Worms
- Baby Healthcare
- Bites & Sting Relief
- Chicken Pox
- Urinary/Bladder Problems
- Vaginal Dryness
-
-
-
- Contraceptive Pill
- Morning After Pill
- Period Delay
- Alternative Contraceptives
- Bacterial Vaginosis
- Period Pain
- Hormone Replacement Therapy (HRT)
- Vaginal Dryness
- PCOS
- Feminine Hygiene Underwear
- Female Hormones Test
- Women's Health - Thrush
- Cystitis Treatments
- Hirsutism (Hair Removal)
- (UTIs) Urinary Tract Infections
-
-
-
-
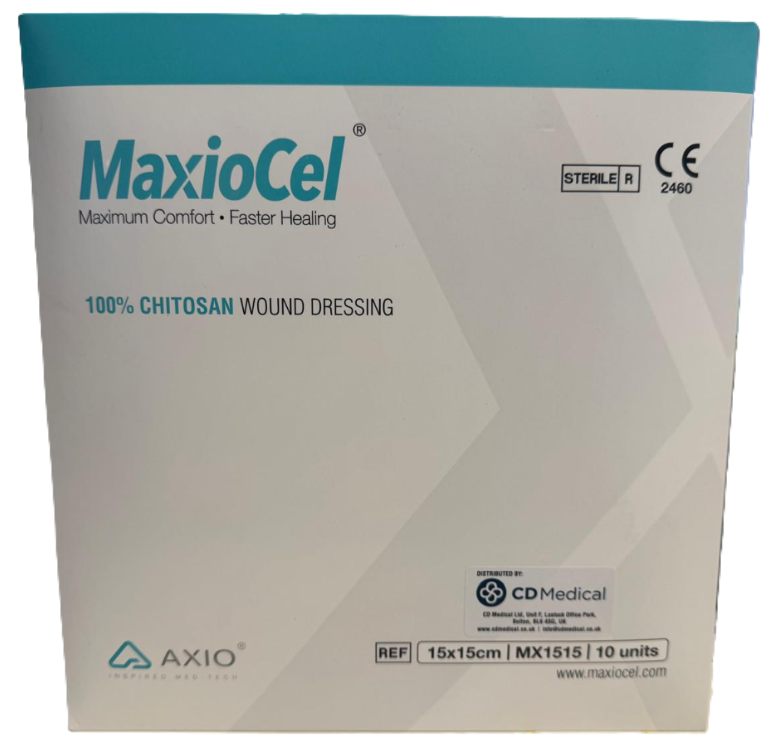
MaxioCel Chitosan Gelling Fibre Wound Dressing | 15cm X 15cm | Pack Of 10
- Categories: Pharmacy Shop Wound Management
Guaranteed Safe Checkout
MaxioCel Chitosan Gelling Fibre Wound Dressing | 15cm X 15cm | Pack Of 10
PRODUCT DESCRIPTION
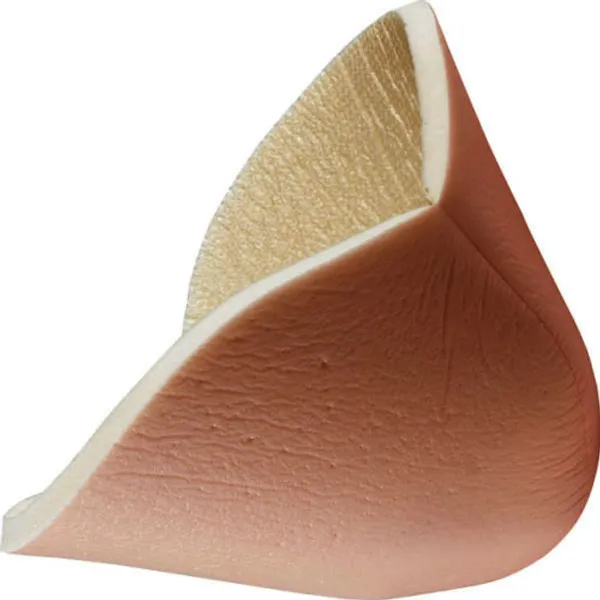
MaxioCel is a sterile, non-woven primary dressing engineered from 100% chitosan fibers. Utilizing Bioactive Microfibre Gelling (BMG) technology, the dressing transforms into a cohesive gel matrix when in contact with wound exudate. This action is designed to sequestrate fluid and maintain the moist environment necessary for natural wound healing.
Derived from purified chitin (shellfish source), the material is processed to remove proteins and is sterilized via gamma irradiation. This 15cm x 15cm format is intended for larger wound areas or can be cut to size by a clinician for specific applications.
Technical Specifications
- Dimensions: 15cm x 15cm
- Material: 100% Chitosan Gelling Fibre
- Technology: Bioactive Microfibre Gelling (BMG)
- Sterility: Gamma Irradiated (Single Use)
- Certifications: Latex-free; Oeko-Tex Standard 100
- Storage: Store between 10–25°C; protect from moisture and heat.
Features & Clinical Application
- Fluid Management: High-capacity absorption designed to retain exudate within the gelling fibers.
- Atraumatic Removal: The cohesive gel structure facilitates one-piece removal via saline irrigation, minimizing trauma to the wound bed.
- Adaptability: Fibers conform to the contours of the wound bed; can be folded or layered for cavity management.
- Skin Compatibility: Dermatologically tested and free from added preservatives.
Important Safety Information (GMC Compliance)
Clinician Oversight Required: This is a medical device. It must only be used following a clinical assessment by a qualified healthcare professional (e.g., GP, Tissue Viability Nurse, or Podiatrist).
Allergy Warning: MaxioCel is derived from shellfish. While purified, it is not recommended for use on individuals with known allergies to chitosan or shellfish. Consult a medical professional before use.
Application: This is a primary dressing. It requires a suitable secondary dressing to be selected based on wound location and exudate levels. Do not re-sterilize or reuse.
BULLET POINTS (Marketplace)
- 100% CHITOSAN FIBRE: Sterile primary dressing utilizing Bioactive Microfibre Gelling (BMG) technology for exudate management.
- COHESIVE GELLING ACTION: Transitions into a gel matrix on contact with fluid, conforming to irregular wound beds.
- EXUDATE RETENTION: Engineered to absorb and retain wound fluid to help protect periwound skin from maceration.
- ATRAUMATIC REMOVAL: Cohesive structure allows for one-piece removal when irrigated with saline.
- LARGE FORMAT: 15cm x 15cm size can be cut or layered to suit various wound depths and shapes.
- PACK OF 10: Individually wrapped, sterile units; latex-free and dermatologically tested.
GOOGLE SHOPPING ATTRIBUTES
- Product Type: Medical Supplies > Wound Care > Wound Dressings
- Condition: New
- Material: Chitosan
- Size: 15cm x 15cm
- Quantity: 10
- Google Product Category: 562